Trending Products Test For Covid-19 - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye
Trending Products Test For Covid-19 - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye Detail:
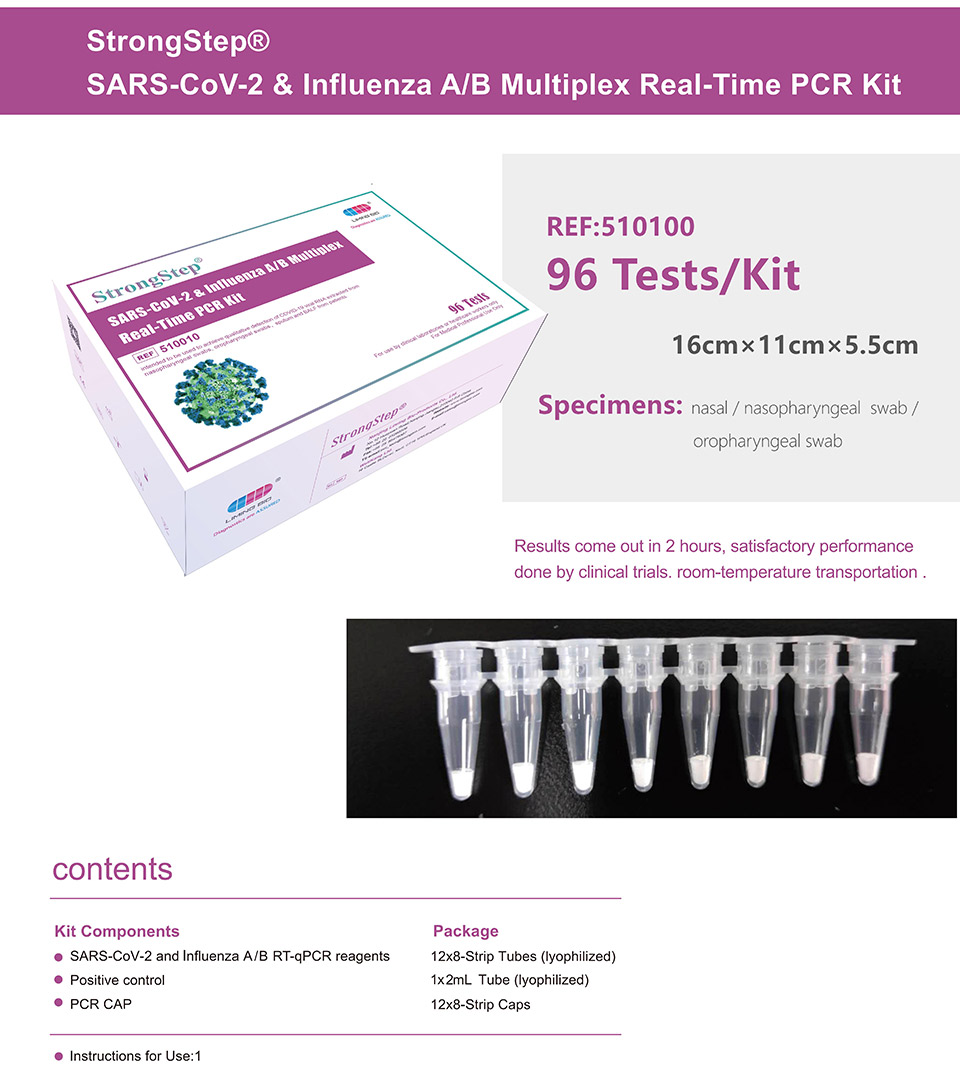
| REF | 510010 | Specification | 96 Tests/Box |
| Detection principle | PCR | Specimens | Nasal / Nasopharyngeal swab / Oropharyngeal swab |
| Intended Use |
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. |
||
The kit is intended for use by laboratory trained personnel
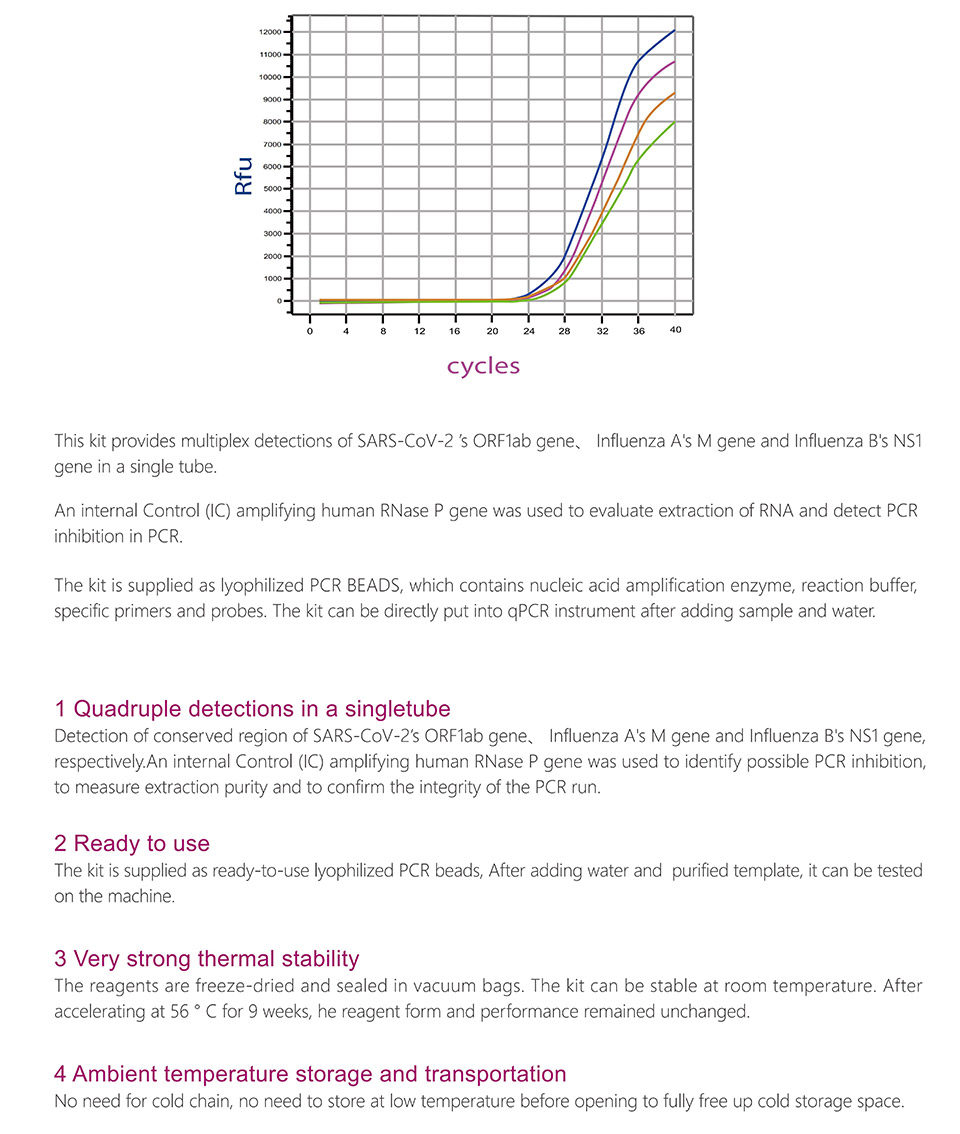
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. RNA from SARS-CoV-2, influenza A, and influenza B is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, influenza A, and/or influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude infection from SARS-CoV-2, influenza A, and/or influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures.
Product detail pictures:
Related Product Guide:
We now have several exceptional workers customers good at marketing, QC, and working with types of troublesome trouble during the creation system for Trending Products Test For Covid-19 - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye , The product will supply to all over the world, such as: Mumbai, Eindhoven, Philippines, We believe with our consistently excellent service you can get the best performance and cost least goods from us for a long term . We commit to provide better services and create more value to all our customers. Hope we can create a better future together.
This is the first business after our company establish, products and services are very satisfying, we have a good start, we hope to cooperate continuous in the future!



