Renewable Design for Covid - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye
Renewable Design for Covid - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye Detail:
| REF | 510010 | Specification | 96 Tests/Box |
| Detection principle | PCR | Specimens | Nasal / Nasopharyngeal swab / Oropharyngeal swab |
| Intended Use |
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. |
||
The kit is intended for use by laboratory trained personnel
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. RNA from SARS-CoV-2, influenza A, and influenza B is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, influenza A, and/or influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude infection from SARS-CoV-2, influenza A, and/or influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures.
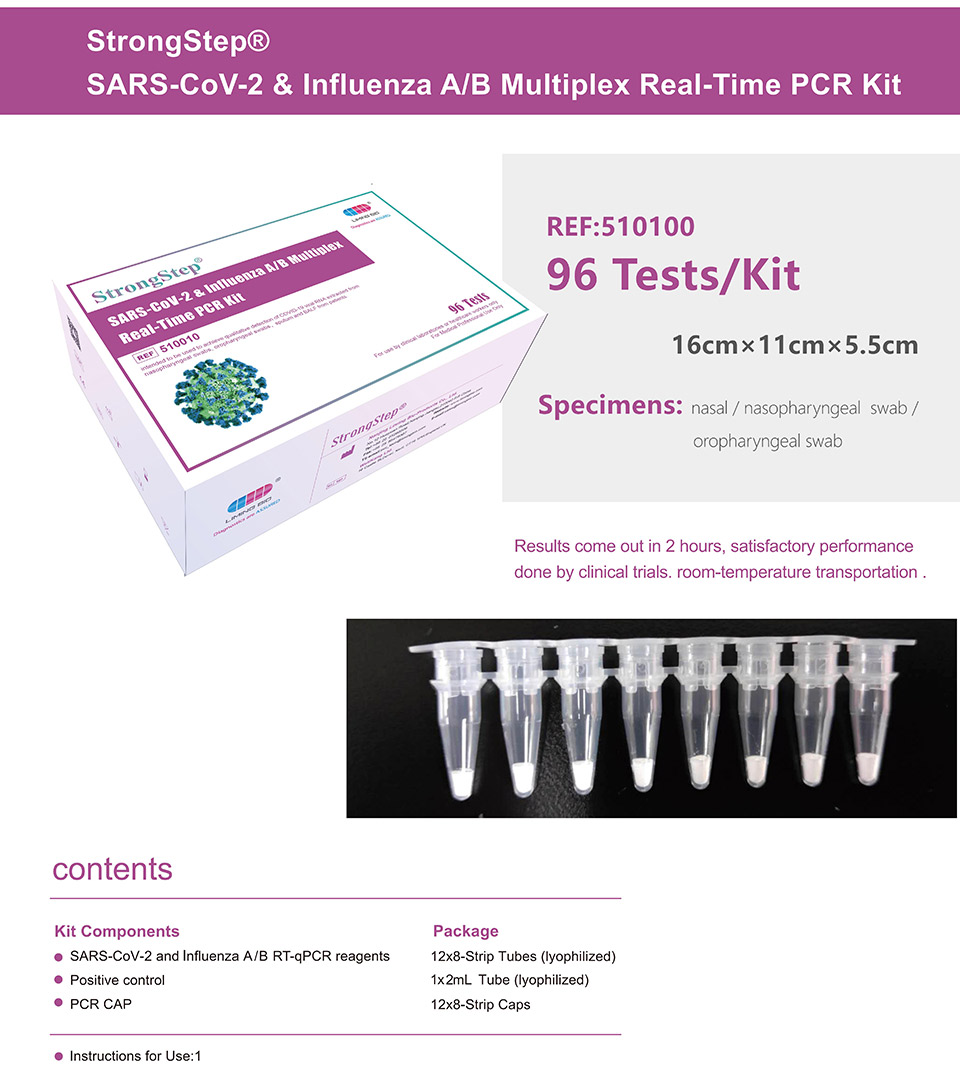
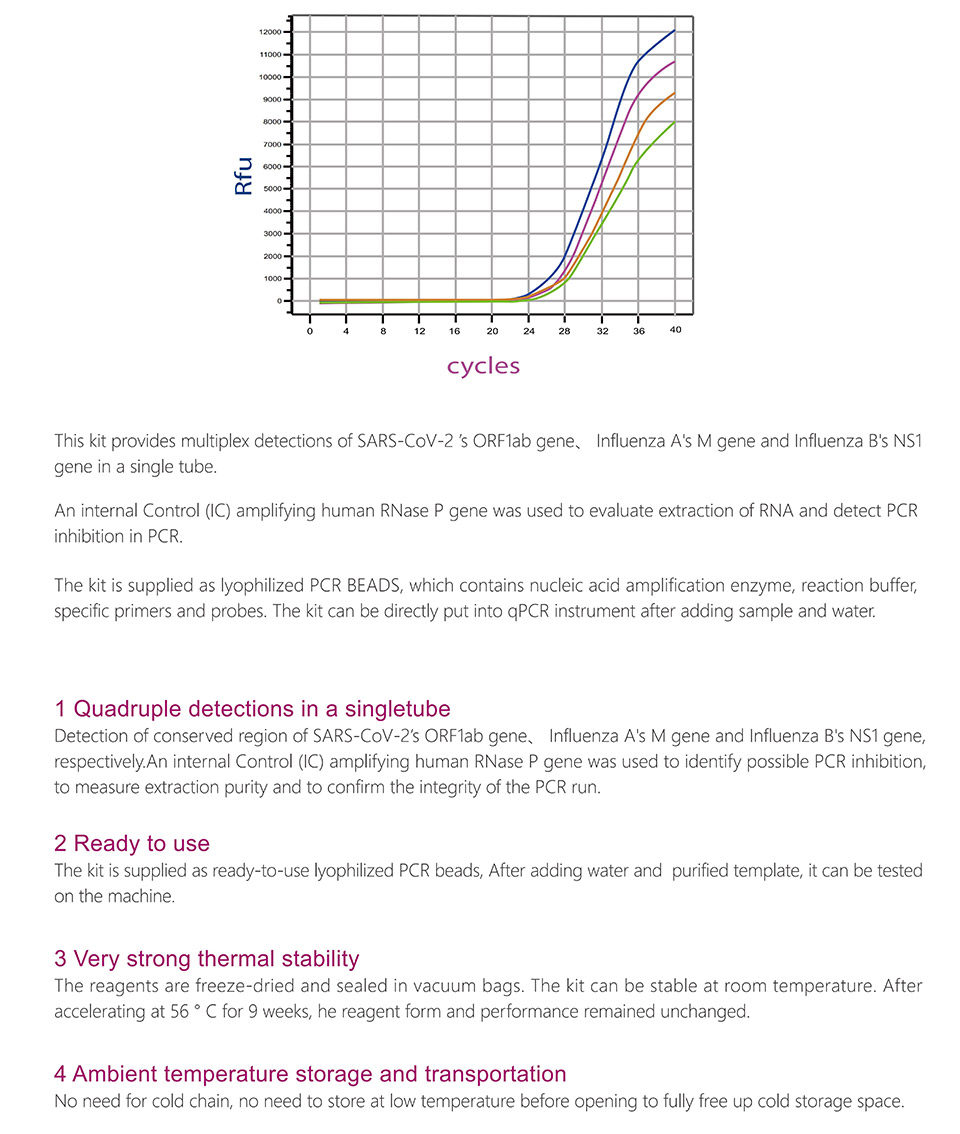
Product detail pictures:
Related Product Guide:
Innovation, excellent and reliability are the core values of our company. These principles today much more than ever form the basis of our success as an internationally active mid-size business for Renewable Design for Covid - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye , The product will supply to all over the world, such as: Madras, Libya, Moldova, When you are keen on any of our items following you view our product list, please feel free to make contact with us for inquiries. You'll be able to send us emails and get in touch with us for consultation and we shall respond to you as soon as we are able to. If it's convenient, you could find out our address in our web site and come to our enterprise. or additional information of our items by yourself. We're generally ready to build lengthy and steady co-operation relations with any possible shoppers within the associated fields.
The company leader recept us warmly, through a meticulous and thorough discussion, we signed a purchase order. Hope to cooperate smoothly