Renewable Design for Covid - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye
Renewable Design for Covid - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye Detail:
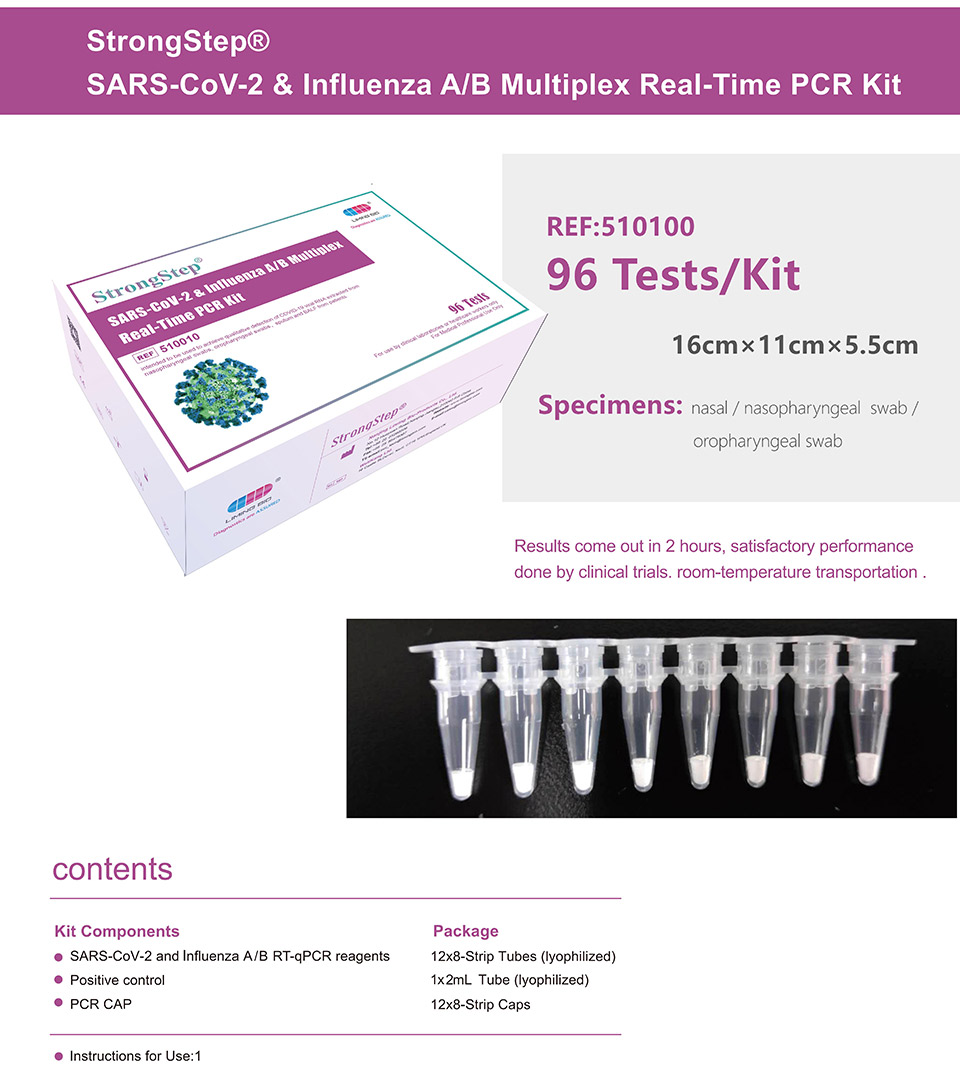
| REF | 510010 | Specification | 96 Tests/Box |
| Detection principle | PCR | Specimens | Nasal / Nasopharyngeal swab / Oropharyngeal swab |
| Intended Use |
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. |
||
The kit is intended for use by laboratory trained personnel
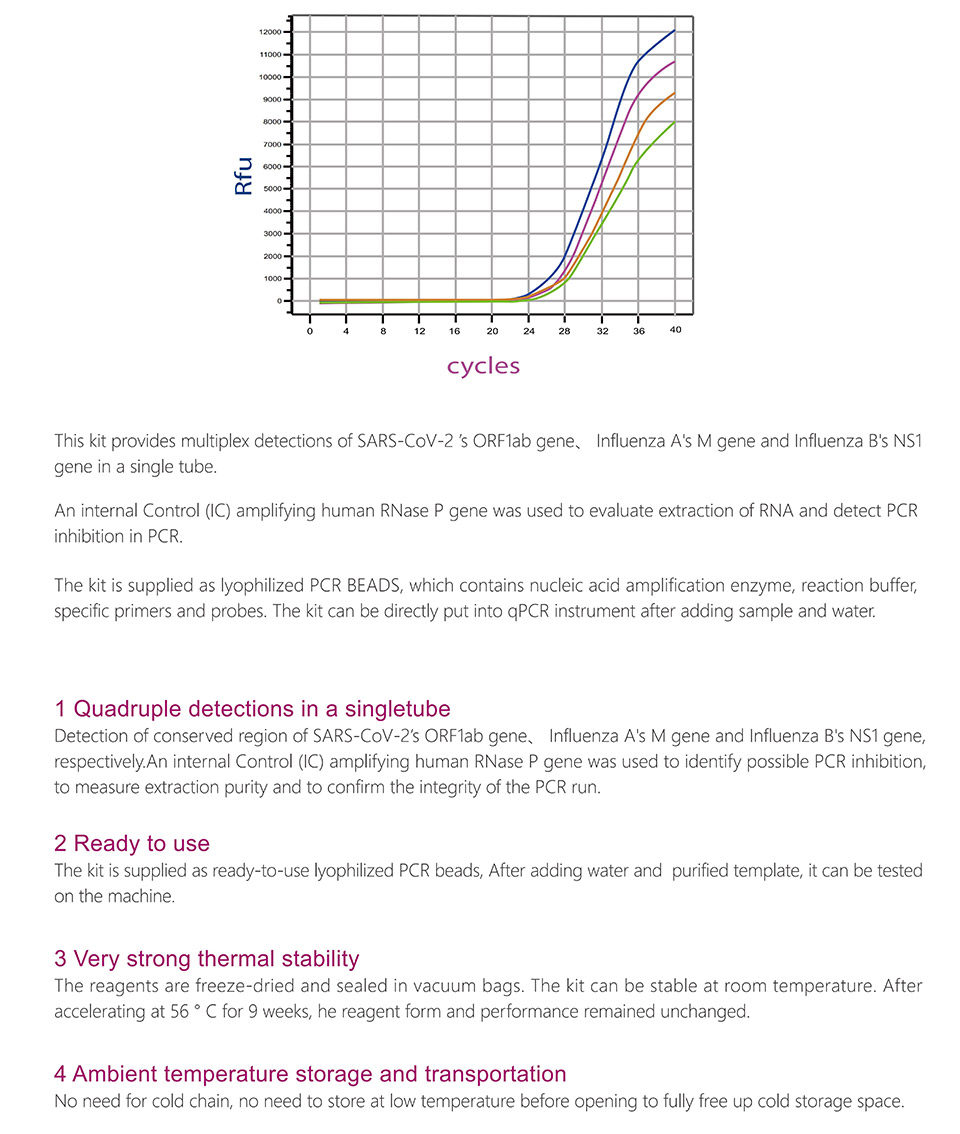
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. RNA from SARS-CoV-2, influenza A, and influenza B is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, influenza A, and/or influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude infection from SARS-CoV-2, influenza A, and/or influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures.
Product detail pictures:
Related Product Guide:
We've got a highly efficient group to deal with inquiries from shoppers. Our purpose is "100% client fulfillment by our product high-quality, price tag & our staff service" and enjoy a superb reputation amongst clientele. With quite a few factories, we will provide a wide variety of Renewable Design for Covid - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye , The product will supply to all over the world, such as: San Diego, Pretoria, Hungary, Our company's main items are widely used all over the world; 80% of our products and solutions exported to the United States, Japan, Europe and other markets. All stuff sincerely welcome guests come to visit our factory.
Staff is skilled, well-equipped, process is specification, products meet the requirements and delivery is guaranteed, a best partner!


