Professional Design Covid-19 Results - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye
Professional Design Covid-19 Results - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye Detail:
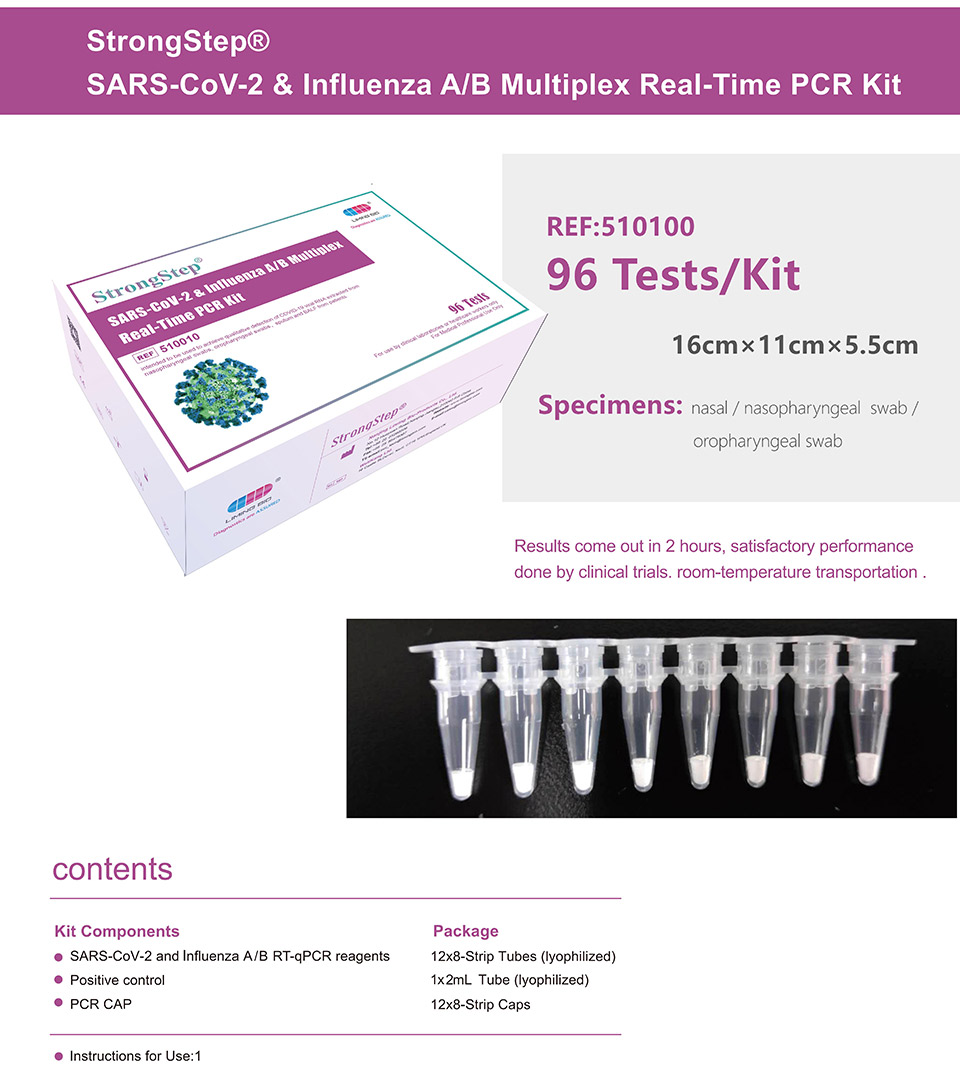
| REF | 510010 | Specification | 96 Tests/Box |
| Detection principle | PCR | Specimens | Nasal / Nasopharyngeal swab / Oropharyngeal swab |
| Intended Use |
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. |
||
The kit is intended for use by laboratory trained personnel
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. RNA from SARS-CoV-2, influenza A, and influenza B is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, influenza A, and/or influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude infection from SARS-CoV-2, influenza A, and/or influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures.
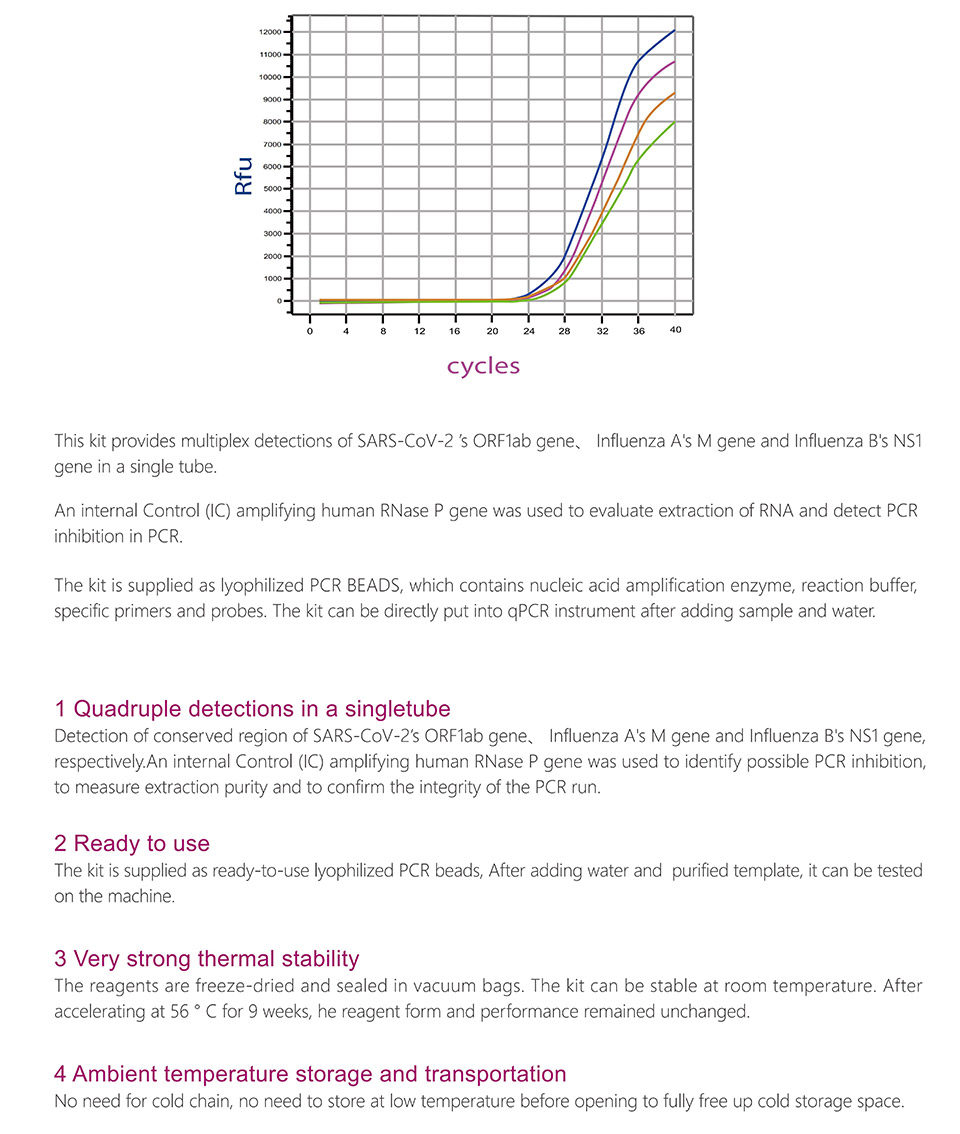
Product detail pictures:
Related Product Guide:
We believe that long term partnership is a result of high quality, value added service, rich experience and personal contact for Professional Design Covid-19 Results - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye , The product will supply to all over the world, such as: Spain, Ukraine, Comoros, We expect to deliver merchandise and services to more users in global aftermarket markets; we launched our global branding strategy by providing our excellent products and solutions all over the world by virtue of our well reputed partners letting global users keep pace with technology innovation and achievements with us.
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!


