Original Factory Saliva Test For Covid Near Me - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye
Original Factory Saliva Test For Covid Near Me - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye Detail:
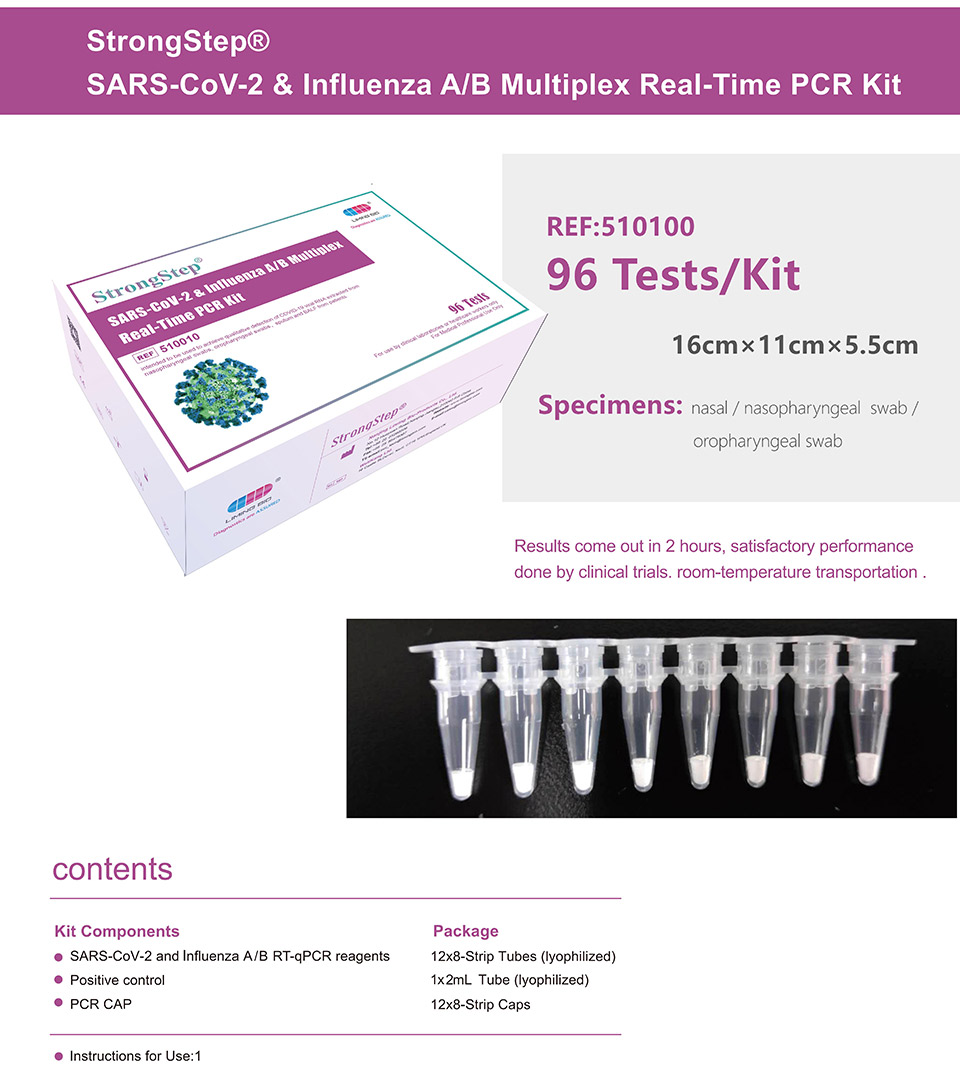
| REF | 510010 | Specification | 96 Tests/Box |
| Detection principle | PCR | Specimens | Nasal / Nasopharyngeal swab / Oropharyngeal swab |
| Intended Use |
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. |
||
The kit is intended for use by laboratory trained personnel
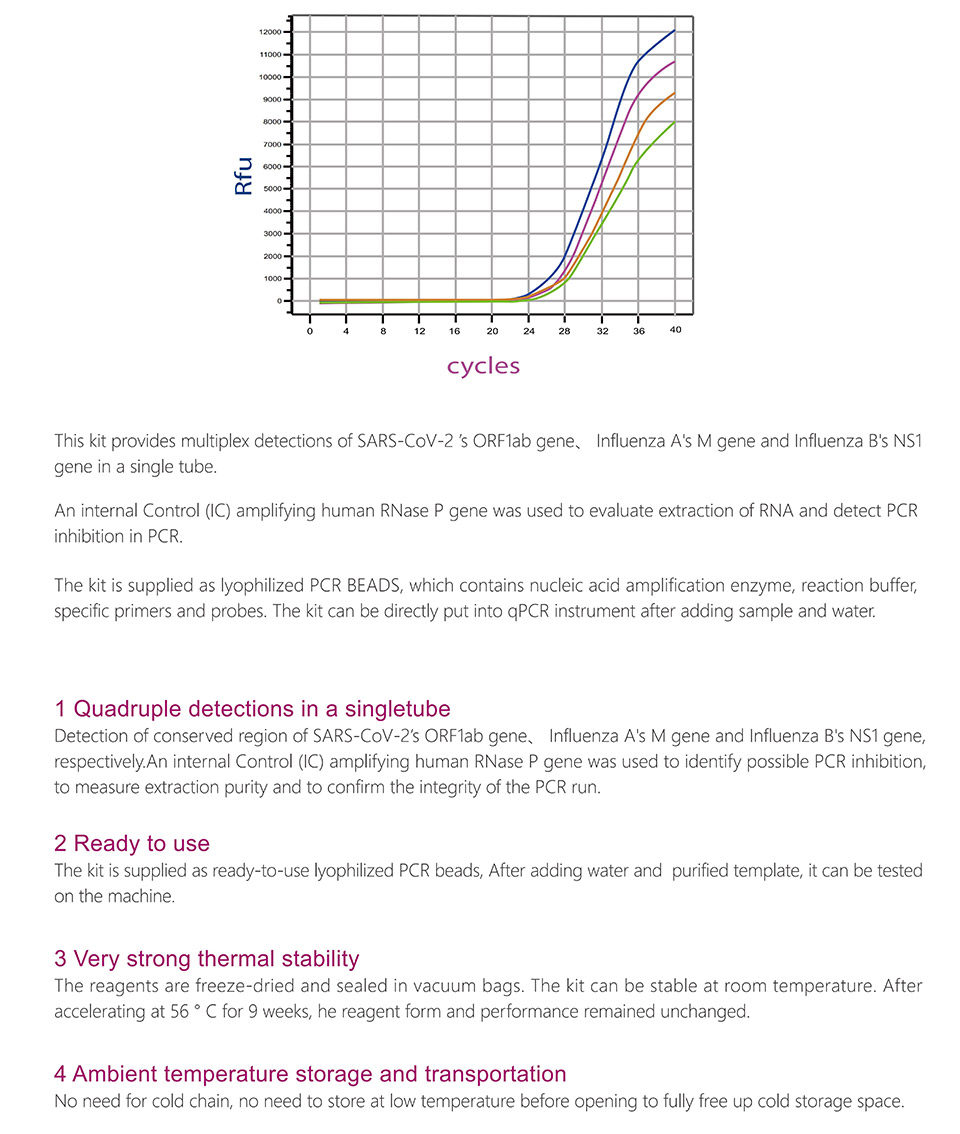
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. RNA from SARS-CoV-2, influenza A, and influenza B is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, influenza A, and/or influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude infection from SARS-CoV-2, influenza A, and/or influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures.
Product detail pictures:
Related Product Guide:
The really abundant projects management experiences and 1 to just one provider model make the high importance of business enterprise communication and our easy understanding of your expectations for Original Factory Saliva Test For Covid Near Me - SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit – Yinye , The product will supply to all over the world, such as: Vietnam, Florence, Curacao, The design, processing, purchasing, inspection, storage, assembling process are all in scientific and effective documentary process , increasing usage level and reliability of our brand deeply, which makes us become superior supplier of the four major product categories shell castings domestically and obtained the customer's trust well.
This is a reputable company, they have a high level of business management, good quality product and service, every cooperation is assured and delighted!



